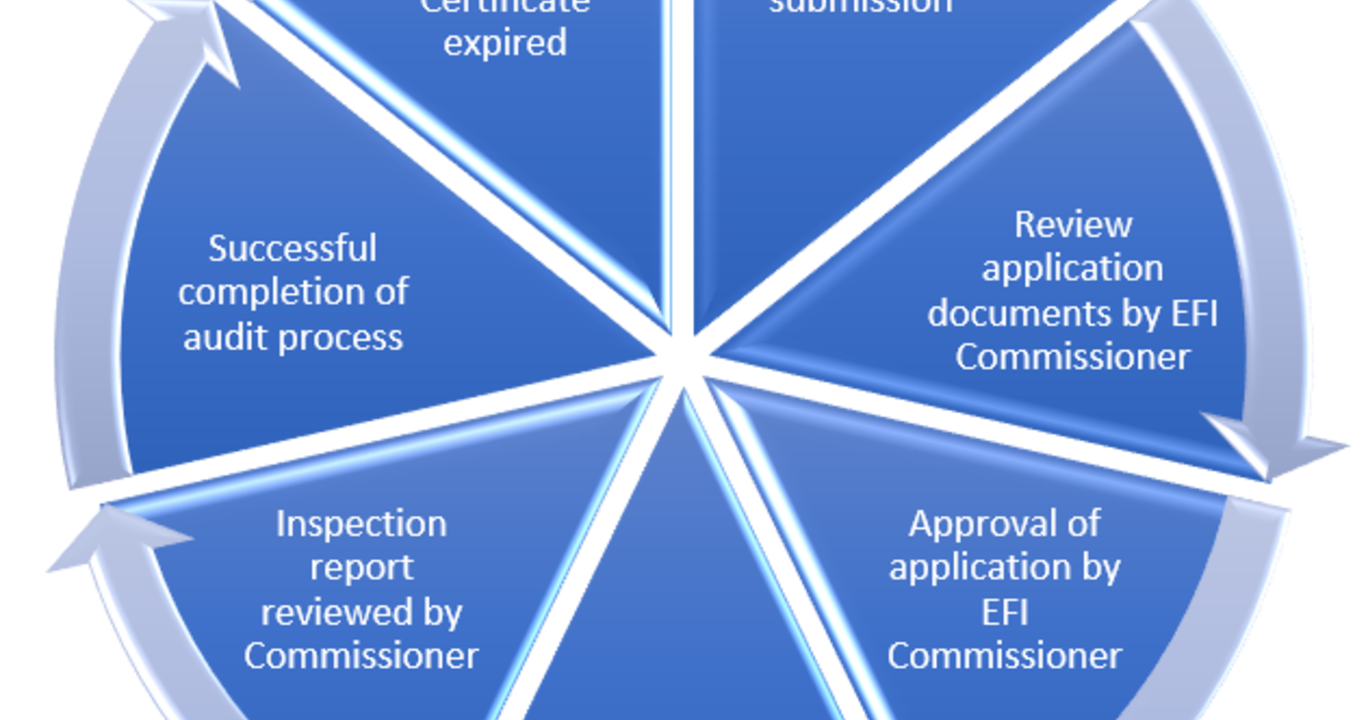
Accreditation
The European Federation for Immunogenetics (EFI) awards the EFI certificate to laboratories that meet the quality requirements set by EFI (Standards). EFI is a European organisation that focuses on immunogenetics, tissue typing and transplantation. The EFI certificate is required by a number of organisations operating in the field of stem cell and solid organ transplantation including JACIE, NMDP and the Eurotransplant foundation.
The EFI Accreditation Programme provides an internationally recognised accreditation scheme for laboratories providing Histocompatibility & Immunogenetics (H&I) testing services in support of solid organ and haematopoietic stem cell transplantation, blood transfusion, disease diagnosis and drug hypersensitivity reactions.
EFI Accreditation update!
On-site inspections for EFI accreditation are currently not being performed due to the restrictions in place because of the covid-19 pandemic. The accreditation cycle for all currently accredited laboratories has been extended with all annual renewals in 2020 now being carried out by document submission and self-inspection. Scheduled inspections will restart in 2021. The Directors of all accredited laboratories have been directly contacted with details of the new application and inspection schedule. This will ensure that all currently accredited labs are able to maintain their accreditation during this period.
Applications by new laboratories will be accepted but there will be a delay in processing these as the process of accrediting a new laboratory cannot be completed without an on-site inspection. The situation is being kept under review by the Accreditation Committee and inspections for new applicants will be scheduled when travel and other restrictions permit. Laboratories should contact Sonja Geelhoed S.H.H.Geelhoed@lumc.nl from the EFI Accreditation Office if they have any questions about a new application already in progress or if they wish to start the process of applying for accreditation.
Requests
Requests for starting the EFI Accreditation
procedure should be sent to:
Sonja Geelhoed
Manager EFI Accreditation Office
+31 71 526 5111
shhgeelhoed@lumc.nl